Green Light for Our Own: How to Achieve Technological Leadership in Pharmaceuticals
On October 7, 2024, at the Gelendzhik Arena, “Development of Innovative Drugs As Condition for Technological Sovereignty of the Russian Federation in Pharmaceuticals” panel discussion was held within the framework of the Biotechmed-2024 Forum. The panel discussion was prepared with the support of ChemRar Group of Companies and the Innovative Pharmaceuticals Committee of the National Champions Association.
Dmitry Galkin, Director of the Pharmaceutical and Medical Industry Development Department, Ministry of Industry and Trade of Russia: “Innovative developments as a bridge from ‘Pharma-2020’ to ‘Pharma-2030’ is one of our three main goals”.
The discussion was attended by key players in the Russian pharmaceutical industry – heads of manufacturing companies, representatives of state authorities, development institutions and scientific teams. The discussion was moderated by Andrey Aleksandrovich Ivashchenko, Doctor of Engineering, Professor of the Russian Academy of Sciences, Chairman of the Board of Directors of ChemRar Group.
The main topic of the discussion was the development of domestic innovative pharmaceuticals, namely, how the creation of own development lines for innovative drugs will help to achieve national goals and technological leadership of Russia by 2030 and in the future up to 2036, as stated in the Decree of the President of the Russian Federation Vladimir Putin dated May 07, 2024.
First of all, the discussion participants noted that by now the main players of the Russian pharmaceutical market have reached a consensus on the key issue: if our industry does not start creating its own innovative drugs, then in the strategic perspective we cannot talk about competition or even about the industry preservation. The main problem is lagging behind global innovations due to the dominance of generic approach in the market. Thanks to the Pharma 2020 Program, we have learned to manufacture all the drugs the country needs, but we are talking only about reproduction. Now we need to direct all our efforts to become an industry that will be able to create its own innovative next- and best-in-class, patent-protected drugs, competitive both in the domestic and global markets in a few years. Only a few companies are doing so now.
According to the Pharma-2030 Strategy, by 2030, domestic manufacturers should reach an indicator of 43% of domestic drugs from the share of the total market in monetary terms, whereas now it is 36%. In essence, it means that the industry strategy preserves the generic development model. During the discussion, the participants discussed, in particular, how to raise the key indicator up to 70% of domestic drugs from the total market share in monetary terms, 30% of which would be innovative high-margin drugs from Russian manufacturers. Whereas, now the majority of the domestic market in monetary terms, is still occupied by foreign Big Pharma, innovations are purchased from foreign countries, primarily unfriendly countries, the profit from which goes to the development of their innovation systems.
In this regard, the question arises, what can really be done to make our industry start not only to copy and reproduce drugs, but also to create new ones in cooperation with Russian science, which there is still a big gap with. Taking into account that Russia has well-established tools and programs to support scientific research, including for subordinate organizations of the Ministry of Education and Science and the Ministry of Health, there is a potential to increase and prioritize them for the development of innovative drugs demanded by the healthcare system.
«We know that access to foreign markets with non-innovative drugs is impossible in principle. But even for innovative drugs, as we have seen through the example of Sputnik V, there are a huge number of regulatory barriers in different countries. Local regulators protect their markets from drugs from other countries, giving priority to their own developers. Russia should have similar mechanisms to support and protect domestic innovations», – emphasized Andrey Ivashchenko, opening the panel discussion.
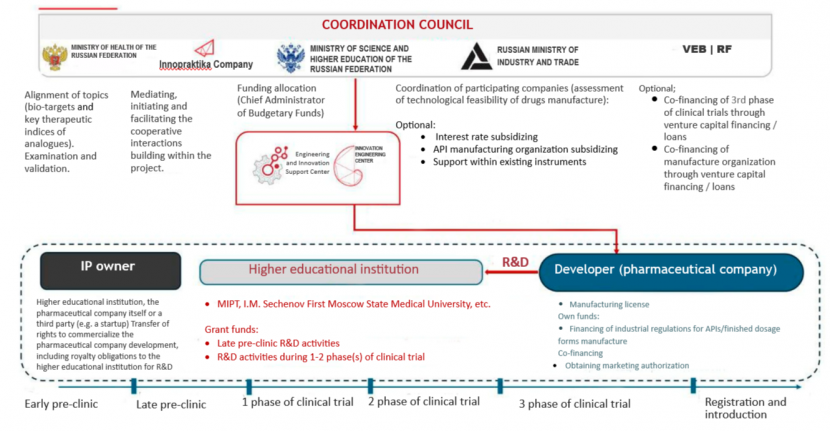
During the session, there was presented an initiative of the Innovative Pharmaceuticals Committee of the National Champions Association, which is being developed with the support of Innopraktika Company together with Innovation Engineering Center, Autonomous Non-Profit Organization and with the participation of the Ministry of Education and Science, the Ministry of Industry and Trade and the Ministry of Health of Russia. The essence of the initiative is to coordinate the work of science, industry, specialized federal executive bodies and subordinate organizations, as well as development institutions in order to, on the one hand, determine priorities, what innovative drugs are necessary for the health care system, taking into account the existing developments around the world and the frontiers, the so-called horizon scanning, without which it will not be possible to ensure technological sovereignty and technological leadership. On the other hand, it is about interdepartmental wells overcoming. The gap between science and industry should be bridged from both sides, both from the regulator and manufacturers, and from the side of science, meaning a part of scientific research, which is now funded by the state, should be reoriented to more applied tasks in the field of pharma, namely to those biotargets, and those technological directions, which will be, among other things, identified as priorities under the proposed “growth completing” initiative.
«If we succeed in this, then maybe we will have a tool that can pull innovations from science to the market and, accordingly, to the healthcare system. This program will allow companies to test a system solution – from demand forecasting to implementation into treatment standards and domestic drugs export in case of success”, – said Andrey Ivashchenko.
According to Aleksey Vinogradov, CEO of Unicorn Capital Partners, innovative drugs of domestic development also need their own separate mechanism or priority in government procurement. «What can be a more vivid evidence of technological leadership than a world-class domestic innovative drug, as a product of Russian science and technology, not copied, not brought from somewhere else, but created here completely. But first, we need to define what an innovative drug of domestic development is, to determine the need for healthcare in the long term, and to guarantee the demand from the state for these drugs for developers. There may be different ways to support innovations during the development process, but it is necessary that an innovative drug developed on a full cycle in Russia should be able to enter the market and, of course, have priority in government procurements. Then the industry will immediately launch the innovation cycle», – the speaker believes.
Kirill Kaem, Deputy Chairman of the Board for Priority Areas of Technological Development of the Skolkovo Foundation, and Vadim Tarasov, Director of the Institute for Translational Medicine and Biotechnology of I.M. Sechenov First Moscow State Medical University, emphasized that a sovereign model in pharma requires expensive R&D-infrastructure. There are individual organizations, usually private, like ChemRar Group, which conduct early exploratory studies, screening, conduct pre-clinics on animals, scale up for clinical trials, but it is not enough. There are tools and state support to build new plants, but it is also necessary to develop the appropriate infrastructure for pre-clinical and clinical trials, screening centers, vivariums, etc. should be created in the country.
Irina Filatova, member of the Committee for Protection of Competition, Head of the Expert Council for the Competition Development in Pharmaceutical Activities of the State Duma of the Russian Federation, supported a comprehensive approach to stimulating the development of innovative medicines, citing examples of the best regulatory practices that have proven their effectiveness in the USA, Europe and China.
In particular, participants of Biotechmed session discussed the practice of “preliminary review” (scientific consultation with the regulator at the research stage), which would allow domestic developers to concentrate their efforts and resources in accordance with the priorities of the domestic healthcare system, increase the efficiency and success of clinical trials, and ensure that innovative drugs become available to patients several years earlier.
About ChemRar Group
ChemRar Group unites research, manufacturing and investment companies in the field of innovative pharmaceuticals to develop and commercialize innovative pharmaceuticals, diagnostics, prophylaxis and new methods of treatment of life-threatening diseases both in Russia and abroad. www.chemrar.ru