Ravidasvir: International experience and Russian perspectives in the fight against hepatitis C
On June 19–20, 2025, Moscow hosted the IV National Expert Council on Viral Hepatitis Elimination—a key industry event that brought together leading specialists in the diagnosis, treatment, and prevention of viral hepatitis from Russia and abroad, as well as representatives of government authorities, pharmaceutical companies, and patient organizations.
The Expert Council reviewed international best practices and prioritized goals and strategies for combating viral hepatitis, as outlined by the WHO. The program featured plenary sessions, expert discussions, and clinical case analyses aimed at developing consolidated solutions to combat viral hepatitis in Russia.
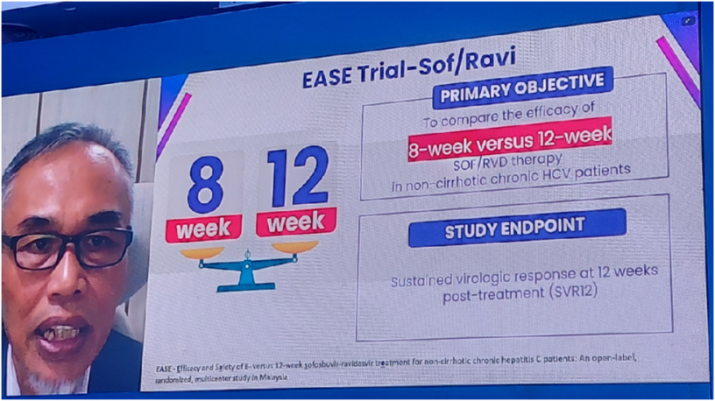
Special attention was given to the treatment of chronic hepatitis C with the innovative drug ravidasvir. Professor Muhammad Radzi Abu Hasan, Director General of the Malaysian Ministry of Health, presented key results from the national hepatitis C program, highlighting the high efficacy of the ravidasvir + sofosbuvir combination (97.6% success rate in patients with genotype 3 and an overall sustained virological response rate of 96.8%).
The findings of a comparative study on 8- and 12-week courses of this therapy were also presented, leading to the approval of an 8-week treatment regimen by Malaysia’s national regulator in May 2025 for patients without cirrhosis.
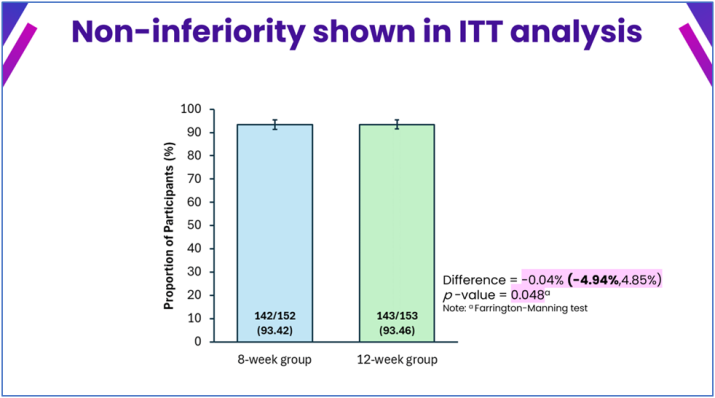
Malaysia’s success demonstrates that with innovative drugs like ravidasvir and comprehensive screening and treatment programs, eliminating hepatitis C is an achievable goal. In 2023, ravidasvir was added to the World Health Organization (WHO) List of Essential Medicines for HCV treatment. “Ravidasvir is a game-changer, offering affordable and effective treatment even for complex cases,” emphasized Professor Radzi.
During the Expert Council, Vladimir Chulanov, Chief Freelance Specialist in Infectious Diseases at the Russian Ministry of Health, announced the successful registration of ravidasvir in Russia. The drug, marketed as CONESCO® (INN: ravidasvir), was developed by the ChemRar Group and registered in December 2024.
CONESCO® is a potent pan-genotypic direct-acting antiviral (DAA) targeting the NS5A protein. It is recommended for adults in combination with sofosbuvir for chronic hepatitis C treatment. The drug has demonstrated efficacy across diverse patient groups, including all HCV genotypes, treatment-naïve and treatment-experienced patients, those with or without cirrhosis, and individuals with HCV/HIV coinfection.
Chronic hepatitis C is an inflammatory liver disease caused by the hepatitis C virus (persisting for six months or longer) that can lead to cirrhosis and primary liver cancer. Viral hepatitis ranks as the third leading cause of infectious disease-related deaths globally, surpassed only by HIV and tuberculosis.
Approximately 71 million people worldwide (1% of the population) live with chronic hepatitis C. In Russia, around 600,000 patients are officially registered, though the actual number may reach several million. In 2022, Russia adopted a national hepatitis C elimination plan aiming to reduce HBV- and HCV-related deaths by 65% and new chronic cases by 90% by 2030.
The introduction of ravidasvir to the Russian market marks a strategic milestone in combating chronic hepatitis C. Early treatment is critical—it prevents severe complications like cirrhosis, liver failure, and hepatocellular carcinoma while reducing viral transmission risks. Timely diagnosis and treatment not only improve recovery rates but also enhance patients’ quality of life, mitigate epidemiological risks, and lower healthcare costs associated with advanced disease management.