Leading scientists note the efficacy of the Russian Avifavir product against COVID-19, including the new Delta and Omicron strains
Avifavir® is the first product approved in Russia for treatment of coronavirus and the world-first registered product using favipiravir as its active ingredient and indicated for COVID-19 treatment.
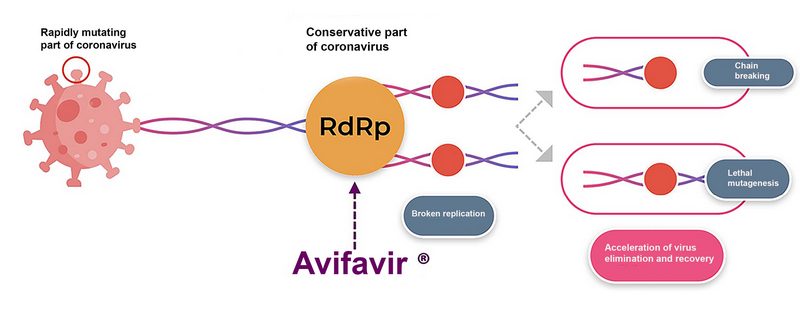
“Avifavir acts on genetically highly conservative RNA virus replication systems, including delta and omicron strains of SARS-CoV-2, and it does so through three complementary mechanisms”, says Konstantin Balakin, PhD (Doctor of Chemistry), head of OrHiMed, Center for Development of New Potential Medicines, a nonprofit partnership of RAS institutions. – Clinical studies have also confirmed the inability of viruses to form resistance to favipiravir, even with prolonged exposure of virus-infected cells to the product. This gives the product a stark advantage not only over highly specific biologics (e.g., antibodies), but also over many other similar nucleoside products prone to inducing rapid evolution of resistant clinical strains. The problem of rapid mutation is particularly characteristic of RNA viruses, since viral RNA polymerase is prone to a high rate of replication errors.
Robert Redfield, a renowned American virologist and director of the U.S. Center for Disease Control and Prevention (2018–2021), notes: “Avifavir has been shown to be effective against COVID-19 in clinical trials and medical practice. Recently, additional direct-action antiviral products have been developed. Studying Avifavir in combination with other antivirals, such as Paxlovid (Pfizer), may offer even better therapeutic options for people at higher risk of COVID-19 disease progression, reduce the risks of drug-resistant virus mutations, and prolong the post-diagnosis window for effective therapy”.
In June 2020, with the support of the Russian Direct Investment Fund (RDIF), ChemRar Group specialists developed and were the first in the world to release Avifavir® (INN: favipiravir), a direct antiviral for COVID-19 treatment, to the Russian and international markets. To develop the product, we used the approach of extending indications of an already established and thoroughly studied broad-spectrum antiviral. This approach is used by scientists around the world to quickly find new treatments, particularly during a pandemic.
Recently, there have been reports of two major pharmaceutical companies preparing for accelerated approval of anti-coronavirus products in EU and the US. For these countries, these are the first tablet dosage forms for the treatment of COVID-19 (only intravenous formulations have been available so far to patients in Europe and the United States). Both products were originally developed for other infectious diseases and are now being investigated for coronavirus as potential extended indication.
This once again proves that Russian scientists were correct in their search for new products to fight the pandemic, which allowed Russian healthcare professionals to obtain access to effective and safe therapies for patients with the new coronavirus infection in the first months of the pandemic in 2020.
During 2020–2021, in addition to the Russian Federation, the favipiravir potential benefits against coronavirus infection were actively studied in more than 50 clinical trials and the product exposure amounted to 4 million patients in China, Japan, EU, Turkey, Iran, Arab states, as well as other countries and regions. Today, the PubMed database of international medical and biological literature contains almost 900 peer-reviewed favipiravir-related papers. At least 700 of them were published in the last 1.5 years. These publications argue for the high efficacy and safety of favipiravir against COVID-19.
Elena Yakubova, Medical Director of ChemRar Group: “Having accumulated extensive experience with Avifavir® in patients with new coronavirus infection from both clinical trials and real-world clinical practice, we see that taking Avifavir® in the first 3–5 days after infection leads to a milder disease in most cases and prevents hospitalization. Over the past 17 months, more than 4 million patients have been treated with favipiravir worldwide. The product was well tolerated with no new adverse events, which confirms the high safety of favipiravir”.
To date, the product has been well studied, and a vast body of information has been accumulated in the scientific literature on various aspects of the favipiravir pharmacology, inlcuding its mechanisms of action, activity in vitro and in vivo, clinical efficacy, safety, cost-effectiveness, potential for combination therapy, analytical control methods, etc. A series of clinical trials in 2020-2021 have provided objective evidence for the efficacy and safety of favipiravir as treatment for COVID-19. If therapy begins in the first days after the onset of disease, the product significantly increases survival rate, reduces viral load, need for artificial ventilation, and length of hospital stay.
References to studies supporting the efficacy of favipiravir against COVID-19:
- In a multicenter randomized phase II/III clinical trial in patients with moderate COVID-19 (NCT04434248), Avifavir provided effective clearance of SARS-CoV-2 virus in 62.5 % of patients within 4 days, was safe and well tolerated (Ivashchenko А.А. et al. Avifavir for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of Phase II/III Multicenter Randomized Clinical Trial. Clin Infect Dis. 2021 Aug 2;73(3):531-534. doi: 10.1093/cid/ciaa1176.). At present there is an opportunity to summarize and supplement both the data of ChemRar Group on the use of the product and the results of its large-scale scientific studies around the world.
- A systematic meta-analysis of 11 clinical trials has demonstrated that favipiravir causes effective clearance of the SARS-CoV-2 virus by day 7 and promotes clinical improvement within 14 days (Manabe et al. Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021; 21(1):489.).
- In a randomized, blind, placebo-controlled phase III study of favipiravir efficacy and safety in 156 COVID-19 patients with moderate-to-severe pneumonia (Japan), the median time to patient recovery was 11.9 days in the favipiravir group and 14.7 days in the placebo group, with a statistically significant difference (p = 0.0136) (Shinkai M. et al. Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial. Infect Dis Ther. 2021 Aug 27; 1-21.).
- Inclusion of favipiravir in the national COVID-19 treatment protocol in Turkey resulted in a pronounced, statistically significant decrease in ICU hospitalization rates from 24 % to 12 % (Guner et al. ICU admission rates in Istanbul following the addition of favipiravir to the national COVID-19 treatment protocol. North Clin Istanb. 2021; 8(2):119.).
- In a retrospective study conducted in specialized public hospitals in Saudi Arabia, the median time to discharge for patients with COVID-19 was 10 days in the favipiravir group compared to 15 days in the maintenance therapy group, across all grades of COVID-19 severity (Alamer et al. Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: a retrospective study with propensity score matching sensitivity analysis/ Curr Med Res Opin. 2021; 37(7):1085.).
- In a randomized, open-label, parallel, multicenter phase III study of 150 patients with mild to moderate COVID-19 conducted in India, the median time to cessation of virus excretion was 5 days versus 7 days, and the median time to clinical recovery was 3 days versus 5 days for favipiravir and control, respectively (Udwadia et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021; 103:62.).
For additional information contact:
Elena Surina
ChemRar Group
PR Director
Mobile +7 9262067871
E-mail: es@chemrar.ru